Therapeutic Potential of Cyanidin-3-Glucoside: A Natural Fatty Acid Amide Hydrolase (FAAH) Inhibitor
1. Introduction
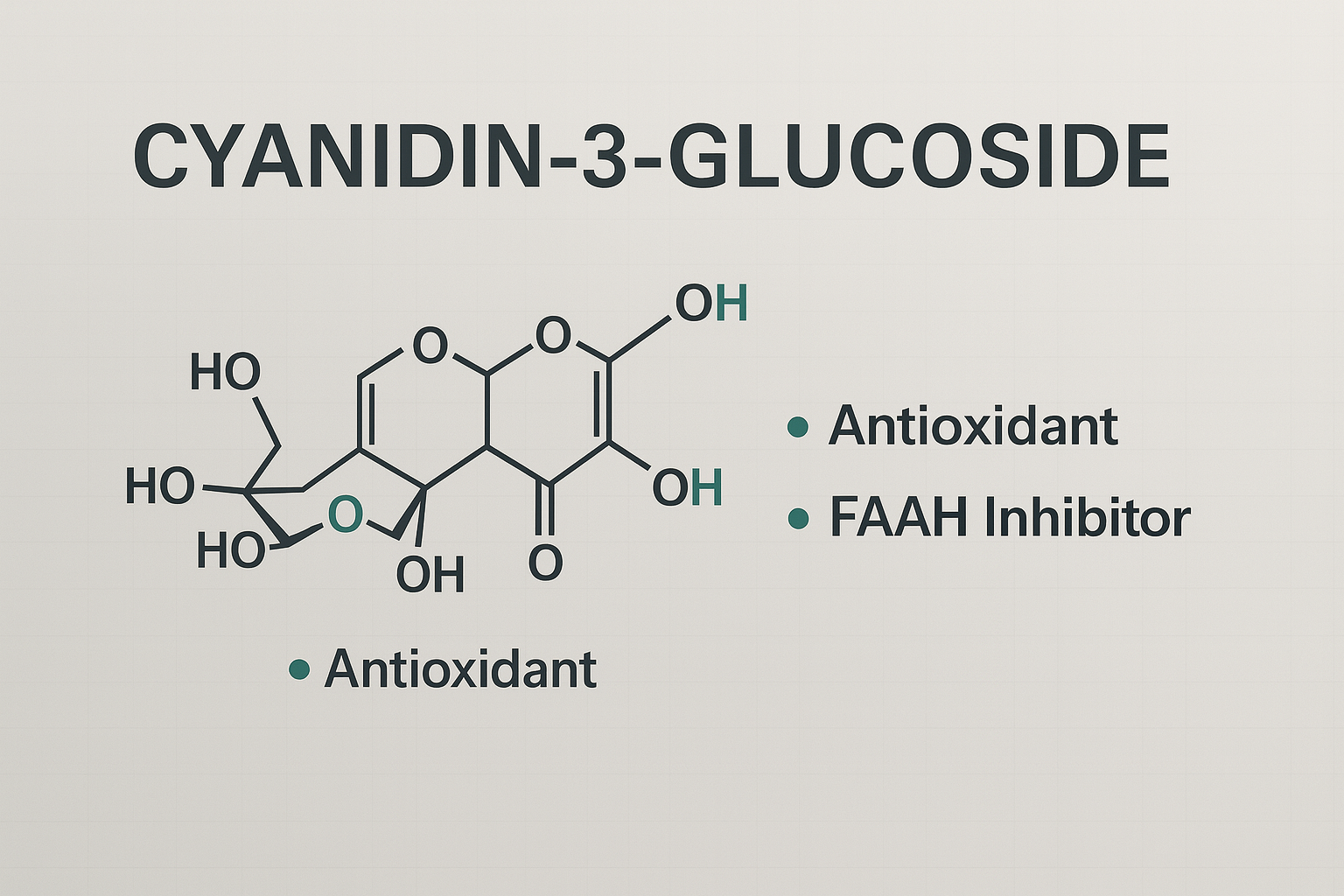
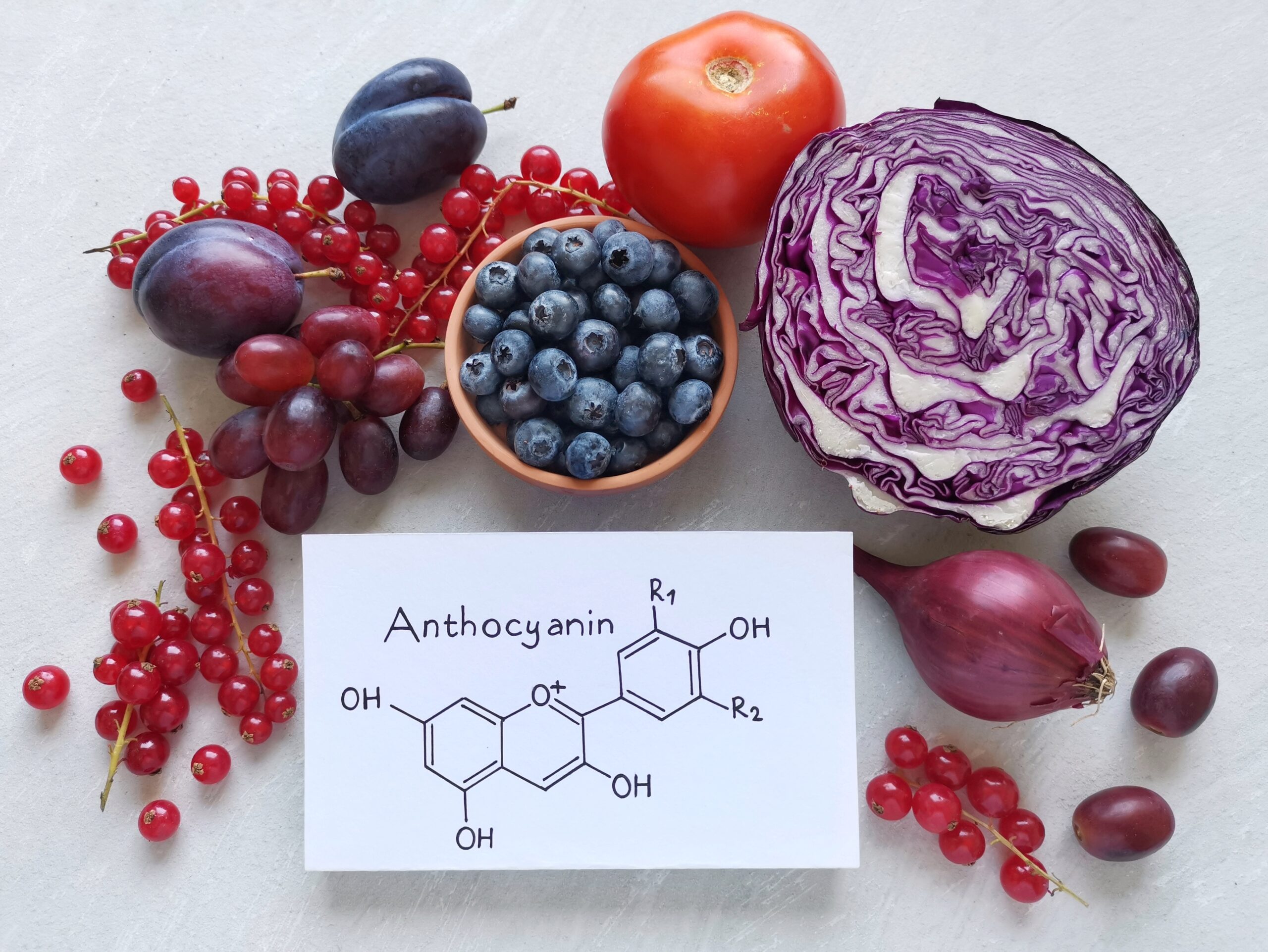
Cyanidin‑3-glucoside (C3G) is one of the most abundant dietary anthocyanins, notably found in berries, purple corn, and black rice. While it is widely recognized for its antioxidant and anti-inflammatory properties, recent research has highlighted its ability to inhibit fatty acid amide hydrolase (FAAH)—the enzyme responsible for degrading the endocannabinoid anandamide (AEA). Inhibition of FAAH sustains AEA signaling, thereby providing therapeutic opportunities in neuroprotection, analgesia, mood regulation, and metabolic health (Butini et al., 2020; Yang et al., 2023).
2. FAAH Inhibition by C3G
In vitro enzymatic assays have demonstrated that C3G inhibits recombinant human FAAH with an IC₅₀ ≈ 152 µM, and achieves approximately 60% maximal inhibition at higher concentrations (Colín-González et al., 2018; Scarpati et al., 2025). This positions C3G as one of the few naturally occurring anthocyanins with documented FAAH inhibitory activity (Colín‑González et al., 2018; Scarpati et al., 2025).
3. Mechanistic Rationale and ECS Modulation
C3G’s flavonoid planar structure is predicted to interact within the FAAH catalytic site via π–π stacking, stabilizing non-productive enzyme–substrate complexes. This mechanism aligns with known modes of FAAH inhibition (Scarpati et al., 2025). More broadly, FAAH inhibition is a validated approach to elevate endogenous AEA and enhance CB₁/CB₂ receptor activation, delivering therapeutic effects without the psychoactive side effects seen with direct agonists (Butini et al., 2020; BMS, 2025).
4. Preclinical Evidence of Therapeutic Effects
Neuroprotection
C3G reduces β‑amyloid and excitotoxic-induced neuronal damage in vitro, improves cognitive outcomes in rodent aging models, and reduces infarct volume after ischemic stroke (Yang et al., 2023; Butini et al., 2020). Sustained AEA mediation through FAAH inhibition likely underlies these neuroprotective benefits via CB₁/B₂ signaling, attenuation of oxidative stress, and reduced excitotoxicity (Butini et al., 2020; BMS, 2025).
Anti‑Inflammatory & Analgesic Actions
C3G suppresses NF‑κB activity, reduces pro-inflammatory cytokine production (e.g. IL‑6, TNF‑α), and decreases nitric oxide generation in immune and vascular cells (Yang et al., 2023). FAAH inhibition enhances CB₂ signaling in immune cells, contributing to anti-inflammatory and analgesic effects (Butini et al., 2020; BMS, 2025).
Metabolic and Cardiovascular Benefits
C3G improves insulin sensitivity in diabetic animal models, enhances endothelial function, and reduces vascular permeability and oxidative stress in metabolic syndrome and obesity contexts (Yang et al., 2023; Scarpati et al., 2025). Endocannabinoid modulation via FAAH inhibition may contribute to improved metabolic homeostasis and cardioprotection.
5. Pharmacokinetics and Safety
C3G is absorbed in intact form in the upper gastrointestinal tract, later metabolized into phenolic derivatives (e.g. protocatechuic acid) that retain bioactivity (Yang et al., 2023). Native C3G exhibits low stability and low oral bioavailability (<1%), but nano-encapsulation and liposomal carriers can enhance systemic delivery significantly (Yang et al., 2023). Toxicology studies show no adverse effects in rodents up to 1,000 mg/kg; early human trials report only mild gastrointestinal discomfort at single doses ≤1 g (Yang et al., 2023).
6. Research Gaps and Future Directions
- Formulation & potency enhancement: The micromolar potency of C3G requires formulation strategies (e.g. nanocarriers) to concentrate its action at the ER membrane where FAAH resides.
- Clinical pharmacodynamics: Human trials measuring changes in plasma/CSF AEA levels post C3G ingestion are lacking.
- Targeted clinical studies: Well-designed trials in mild cognitive impairment, chronic pain, metabolic syndrome, and neuroinflammation are needed to confirm therapeutic utility.
7. Conclusion
Cyanidin‑3‑glucoside offers a naturally derived strategy to modestly inhibit FAAH, elevating endocannabinoid signaling while also delivering antioxidant, anti-inflammatory, and cardiometabolic benefits. Its dietary accessibility and favourable safety profile suggest strong potential for nutraceutical or adjunct therapeutic applications. Nonetheless, improvements in bioavailability and translational clinical evidence are essential to unlock its full therapeutic promise.
Disclaimer: This article is for informational purposes only and is not intended to replace medical advice. Always consult with a healthcare professional before beginning any new supplement, especially if you are on medication or managing a health condition.
References
Butini, S., Gemma, S., & Campiani, G. (2020). Natural compounds and synthetic drugs to target FAAH enzyme. In M. Maccarrone (Ed.), New tools to interrogate endocannabinoid signalling: From natural compounds to synthetic drugs (pp. 337–384). Royal Society of Chemistry.
Colín‑González, A. L., Scarpati, F., & Durán, A. P. (2018). Cyanidin‑3‑O‑glucoside inhibits different enzymes involved in central nervous system and metabolic diseases: In vitro and in silico studies. South African Journal of Botany, 119, 77–85. https://doi.org/10.1016/j.sajb.2018.07.001
Scarpati, F. et al. (2025). Phenolic cyanidin‑3‑O‑glucoside: Purification and evaluation against FAAH. Food & Function, advance online publication. https://doi.org/10.1016/S2212-4292(25)01299-4
Yang, M., Ahmad, N., Hussain, M., & Guan, R. (2023). A review of recent advances on cyanidin‑3‑O‑glucoside: The biotransformation, absorption, bioactivity and applications of nano‑encapsulation. Food & Function, 14(7), 551. https://doi.org/10.1039/d2fo03824b
BMS. (2025). Endocannabinoid signaling and the role of FAAH and MAGL. BMS Scientific Media Resources.