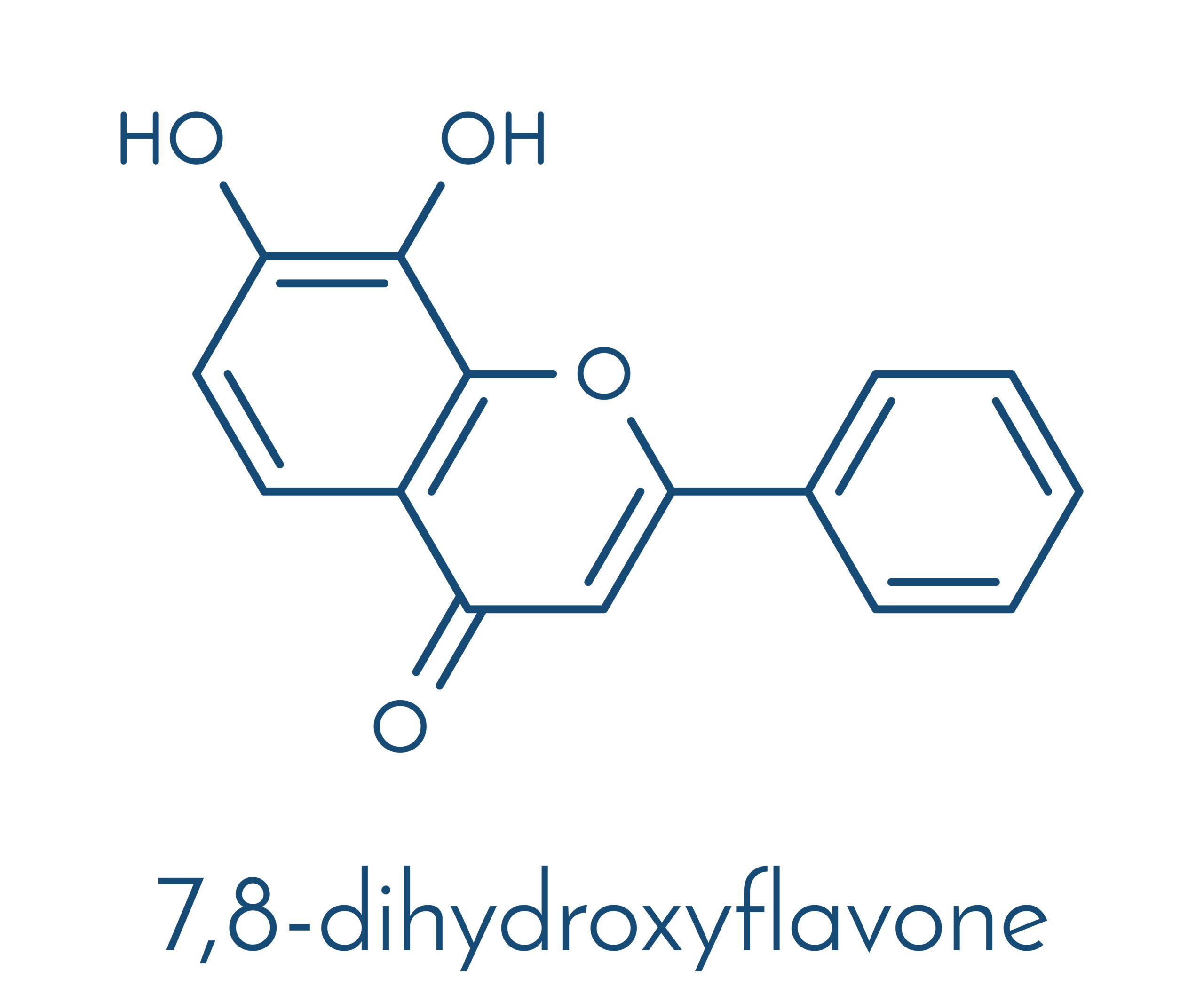
Neuroprotective Effects of 7,8-Dihydroxyflavone in Traumatic Brain Injury: Attenuation of Neuronal Death via TrkB Receptor Activation
Introduction
Traumatic brain injury (TBI) is a major cause of death and long-term neurological disability worldwide. It initiates a cascade of primary and secondary injury processes, including oxidative stress, excitotoxicity, inflammation, and neuronal apoptosis. While the primary mechanical injury is often unavoidable, therapeutic strategies targeting the secondary injury mechanisms have shown promise in limiting neuronal loss and improving functional recovery.
Brain-derived neurotrophic factor (BDNF) and its receptor, tropomyosin receptor kinase B (TrkB), play crucial roles in neuronal survival, plasticity, and repair. However, BDNF itself faces pharmacokinetic limitations, such as poor blood-brain barrier (BBB) penetration and rapid degradation. 7,8-Dihydroxyflavone (7,8-DHF), a small-molecule flavonoid, has emerged as a potent TrkB agonist capable of mimicking BDNF’s neuroprotective effects with superior bioavailability.
This article reviews current evidence supporting the use of 7,8-DHF in attenuating neuronal death following TBI, focusing on its mechanisms of action via TrkB receptor activation.
Mechanism of Action: 7,8-DHF as a TrkB Agonist
7,8-DHF is a naturally occurring flavone that binds to the extracellular domain of TrkB receptors, triggering receptor dimerization and autophosphorylation. This activation leads to downstream signaling through pathways such as:
- PI3K/Akt pathway – promotes cell survival and inhibits pro-apoptotic proteins.
- MAPK/ERK pathway – supports neurogenesis and synaptic plasticity.
- PLCγ pathway – involved in calcium signaling and synaptic modulation.
These pathways contribute collectively to cellular resilience, axonal regeneration, and anti-apoptotic responses after brain injury.
Evidence from Preclinical Models
1. Attenuation of Neuronal Apoptosis
Several rodent studies have demonstrated that 7,8-DHF significantly reduces neuronal apoptosis in TBI models. Zeng et al. (2012) showed that intraperitoneal administration of 7,8-DHF post-injury decreased caspase-3 activation and neuronal cell death in the cortex and hippocampus. The reduction in TUNEL-positive cells correlated with increased TrkB phosphorylation and Akt activation.
2. Reduction of Oxidative Stress and Inflammation
Oxidative damage and neuroinflammation exacerbate neuronal injury after TBI. Studies indicate that 7,8-DHF administration leads to decreased levels of malondialdehyde (MDA), a marker of lipid peroxidation, and enhances the activity of antioxidant enzymes like superoxide dismutase (SOD). Moreover, 7,8-DHF treatment downregulates inflammatory cytokines (e.g., TNF-α, IL-1β) and microglial activation, as reported by Liu et al. (2014).
3. Preservation of Cognitive Function
TBI often results in lasting cognitive impairments, particularly in memory and executive function. In behavioral assays such as the Morris water maze and novel object recognition test, rodents treated with 7,8-DHF displayed significantly better performance compared to untreated controls. These findings suggest that TrkB activation not only preserves neuronal viability but also facilitates cognitive recovery.
Pharmacokinetics and Safety Profile
7,8-DHF demonstrates high oral bioavailability and effective penetration across the BBB, distinguishing it from BDNF and many neurotrophic agents. Pharmacokinetic studies have confirmed that 7,8-DHF reaches therapeutic concentrations in the brain within 30 minutes of systemic administration. Additionally, subchronic dosing in animals has not shown major toxicological effects, supporting its potential for therapeutic use.
Clinical Translation: Opportunities and Challenges
While preclinical results are promising, clinical studies on 7,8-DHF in human TBI are currently lacking. Key considerations for translation include:
- Dosing and timing: Identifying the therapeutic window and optimal dosage for neuroprotection.
- Long-term safety: Assessing potential side effects of chronic TrkB activation.
- Combination therapies: Evaluating synergistic effects with other neuroprotective agents or rehabilitation strategies.
Furthermore, derivatives and analogs of 7,8-DHF are under investigation to enhance stability and minimize metabolic degradation.
Conclusion
7,8-Dihydroxyflavone offers a compelling therapeutic approach for mitigating secondary neuronal damage in TBI through TrkB receptor activation. By mimicking BDNF signaling, it reduces apoptosis, inflammation, and cognitive dysfunction in preclinical models. Future clinical research is warranted to evaluate its efficacy, safety, and practical application in human populations. If successfully translated, 7,8-DHF could represent a significant advancement in the management of traumatic brain injury and other neurodegenerative conditions.
Disclaimer: This article is for educational purposes only and does not substitute for professional medical advice. Always consult a healthcare provider before starting any supplement protocol.
References
Chen, C., Wang, Z., Zhang, Z., Liu, X., & Ye, K. (2018). 7,8-Dihydroxyflavone protects synaptic function and reduces hippocampal neuroinflammation in traumatic brain injury. Journal of Neuroinflammation, 15, 1–12.
Zeng, Y., Liu, Y., Wu, M., Liu, J., & Hu, Q. (2012). 7,8-Dihydroxyflavone attenuates traumatic brain injury-induced neuronal apoptosis via activation of TrkB. Brain Research, 1454, 11–22.
Liu, X., Chan, C. B., Jang, S. W., & Ye, K. (2014). 7,8-Dihydroxyflavone protects against impairments in learning and memory after traumatic brain injury in mice. Neurobiology of Disease, 74, 365–373.